After a clinical trial ends, the results are often peer reviewed before being published in a scientific journal to ensure accuracy and integrity. This means that other medical experts who were not involved with the study will examine the results before it’s published to ensure that the conclusions are accurate.
If the results are published, the two main ways to access the results of a clinical trial are to:
- Find them online from publicly available databases.
- Contact the company that sponsored the clinical trial.
Regardless of what the results are, the outcome of a clinical trial should be published so that others can use the information to help them make decisions about treatment and healthcare; however, this doesn’t always happen. Unfortunately, over half of results aren't published, because it’s often the case that only studies where the treatment was successful in treating patients will be published. Another reason a study may not have results available is if the trial ended before it was completed.
If you participated in a clinical trial, you should be able to get the results of the study whether or not they are published.
How to find the results online
Search in the Pubmed database
Many published studies are accessible through the Pubmed, which is an online resource maintained by the US National Library of Medicine. Here, you can access thousands of scientific articles for free and search for the results using the name of the study, condition, treatment, or Protocol ID number.
Scientific papers about a clinical trial usually include:
-
General information and history about the medical condition
-
An explanation of how the study was conducted
-
An overview of the results
-
An interpretation of the results and why they’re important
Unfortunately, many publications are not available for free in this database and require payment, which can be very expensive. Some are available for free, and you can ask your local librarian about getting full-text articles through interlibrary loans. If you participated in the clinical trial, you should reach out to a member of the research team, who may be able to get you access to the publication for free. Additionally, if the results are particularly important, they may be featured in the news and discussed by patient advocacy groups before or after they are published in a scientific journal.
Find the results from ClinicalTrials.gov
ClinicalTrials.gov is a government resource maintained by the National Library of Medicine (NLM) at the National Institutes of Health (NIH), that provides the public with access to information on publicly and privately supported clinical studies. Information on ClinicalTrials.gov is provided and updated by the sponsor or principal investigator of the clinical study.
Here's a step by step guide on how to find the result of a study on ClinicalTrials.gov:
-
Go to the Advanced Search page from their homepage
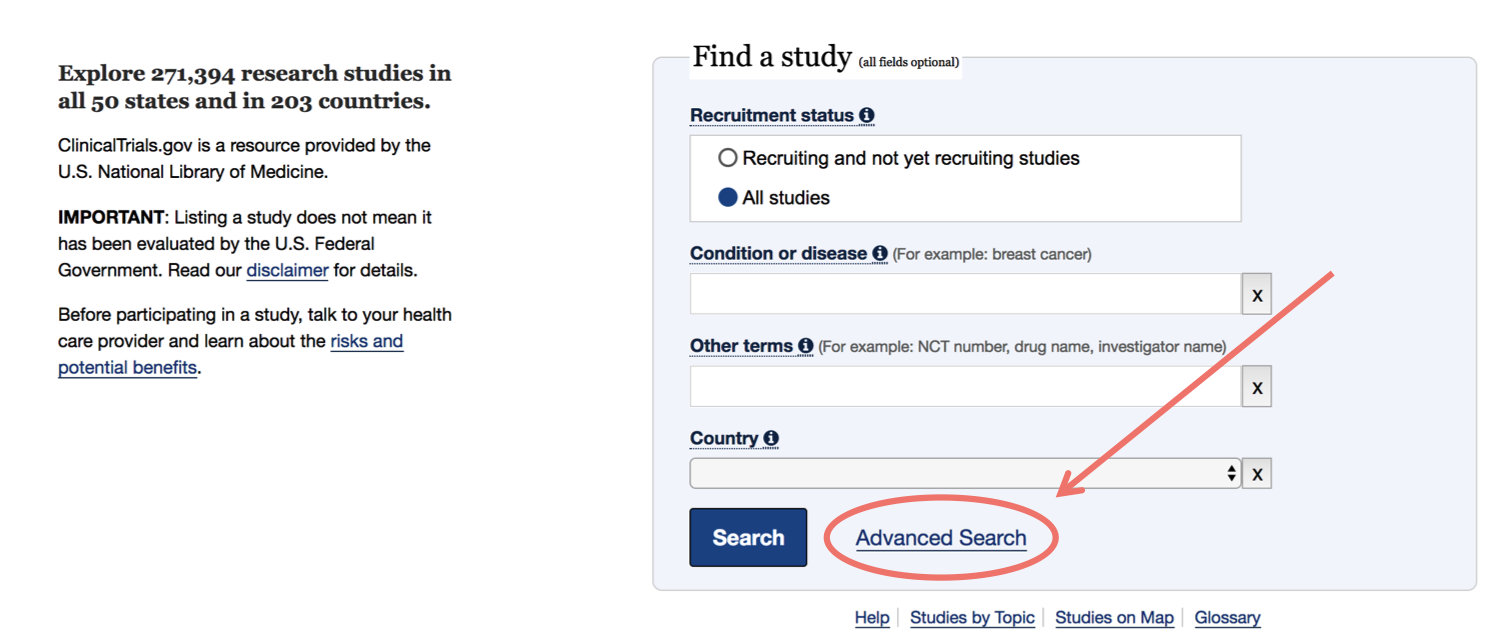
-
On the advanced search page, change the Study Results option from "All Studies" to "Studies With Results".
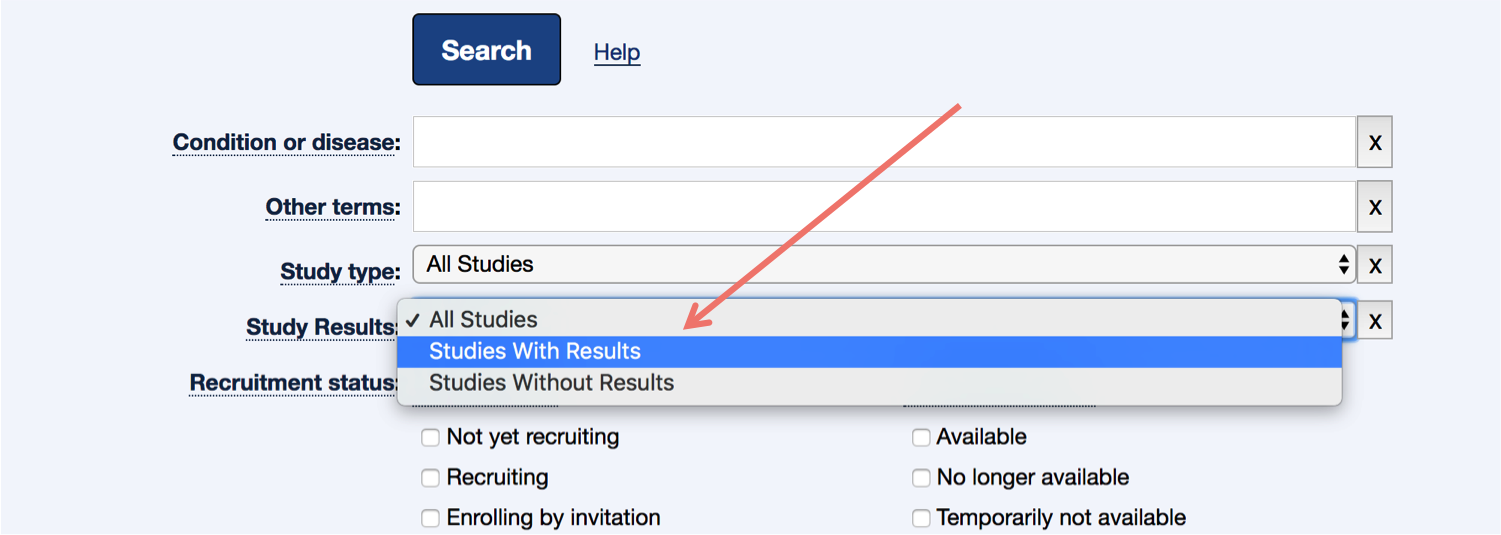
-
If you're looking for a specific study's results, you can narrow down the trials in the Other Terms box.
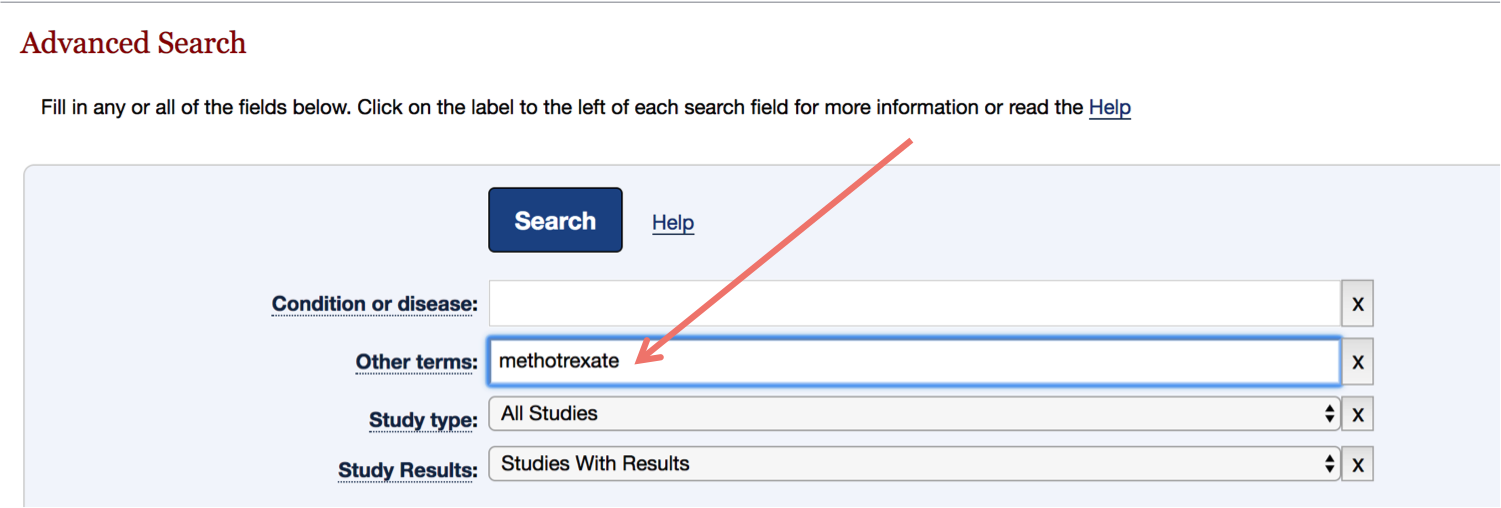
Here are a few different terms you can type into the search box to find the page for a specific clinical trial:
- The name of the study, like "Comparison of Lasers in the Treatment of Scars"
- The name of the treatment, such as "methotrexate"
- The name of the sponsor (organization that funded the trial), like "AbbVie"
- Once you're on the page for the study you're interested in, click on the 'Study Results’ tab (if the results are not available, the tab won't be there).
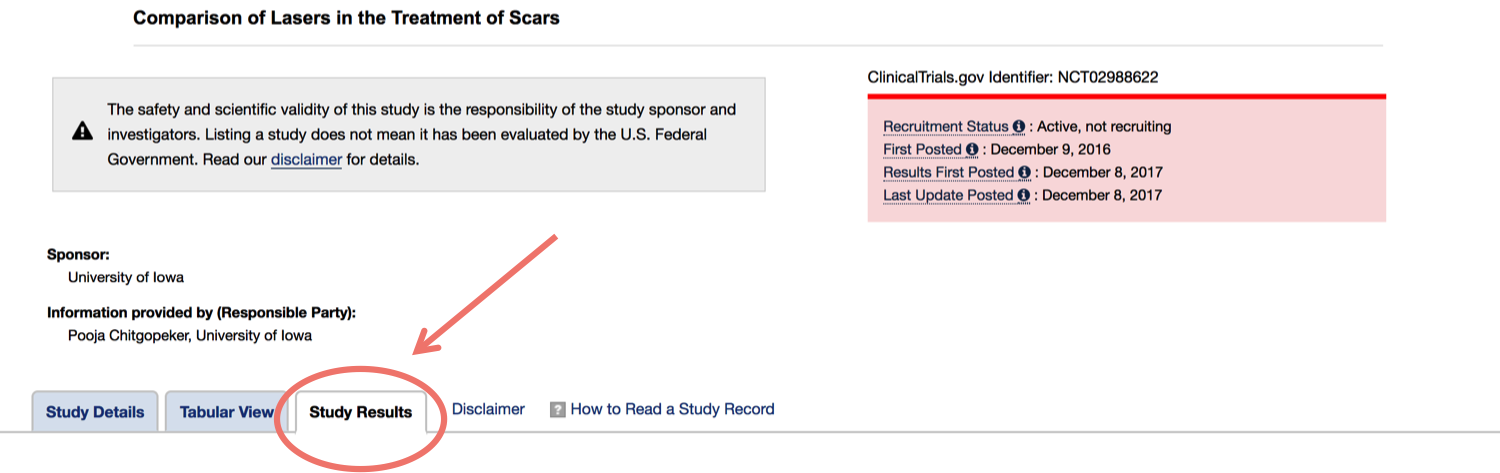
On the study results section, you’ll be able to find any released information about how many people participated in the trial, statistical results, and answered research questions.
If you navigate onto the Study Details tab, under the More Information section, you'll also be able to find relevant published papers about the study.
Although nearly 90% of trials in the database don't have results, you may be able to access the results of a clinical trial through them. Some studies will ask the FDA to delay posting the results of a trial because they have to perform additional statistical tests, or don’t want to make their results public for some reason. These delays can last years, which can make accessing your results through that method less reliable. In 2016, the Department of Health and Human services passed a rule to require that all NIH sponsored clinical trials be reported on ClinicalTrials.gov - so if you're looking for results of a trial from the NIH, it will likely be posted there.
Note: The personal identity of participants will never be included in papers or news coverage.
Contacting the sponsor of the trial
If you can't find the study results online, it may be possible to access them by contacting the sponsor of the study. The sponsor is the individual, organization, or company that funded the clinical trial.
Searching for the name of the sponsor + clinical trial results will typically bring up the information about where their clinical trial results can be found, or how to contact them to learn more. For example, this is Pfizer's page that outlines steps on how to access all of their clinical trial results. Some study sponsors have study results available from their own websites, such as the National Cancer Institute's clinical trial results blog.





