Journey to a rare diagnosis
“Every college student has chronic fatigue.”
An infuriating phrase, Jamie Koll, a 28 year old New York City dweller, event planner, famous foodie instagrammer (@girlswhoeat) and rare disease patient advocate heard from physicians when she tried to seek care for the digestive issues she struggled with throughout high school and college. Layer comments like that alongside a slew of digestive misdiagnoses including pelvic floor syndrome, IBS-C, and swallowing air and Jamie was at her wits end.
After a meal out with her friend in 2018 that ended in the inability to breathe or swallow properly, Jamie knew in her gut something wasn't right. She booked an appointment with a gastroenterologist and for the first time after five years of searching for answers, the doctor finally decided to order a CT scan that illuminated the way to a diagnosis.
The scan revealed that Jamie’s spleen was seventeen times the size it should be and her liver was double the size. Concerned that it could be lymphoma, within 24 hours, Jamie was set up to work with a hematologist. After receiving “every test under the sun” over the span of two months, a DNA sequencing confirmed that she had Gaucher Disease Type 1, an inherited, rare, genetic lysosomal storage disorder that affects many of the body’s tissues and organs and can result in bone abnormalities, lung disease, anemia, enlargement of spleen and liver, and easy bruising. Jamie was one of the first patients to ever be diagnosed through GI symptoms as it is typically diagnosed after complaints of bone pain or fractures.
Patients Have Power
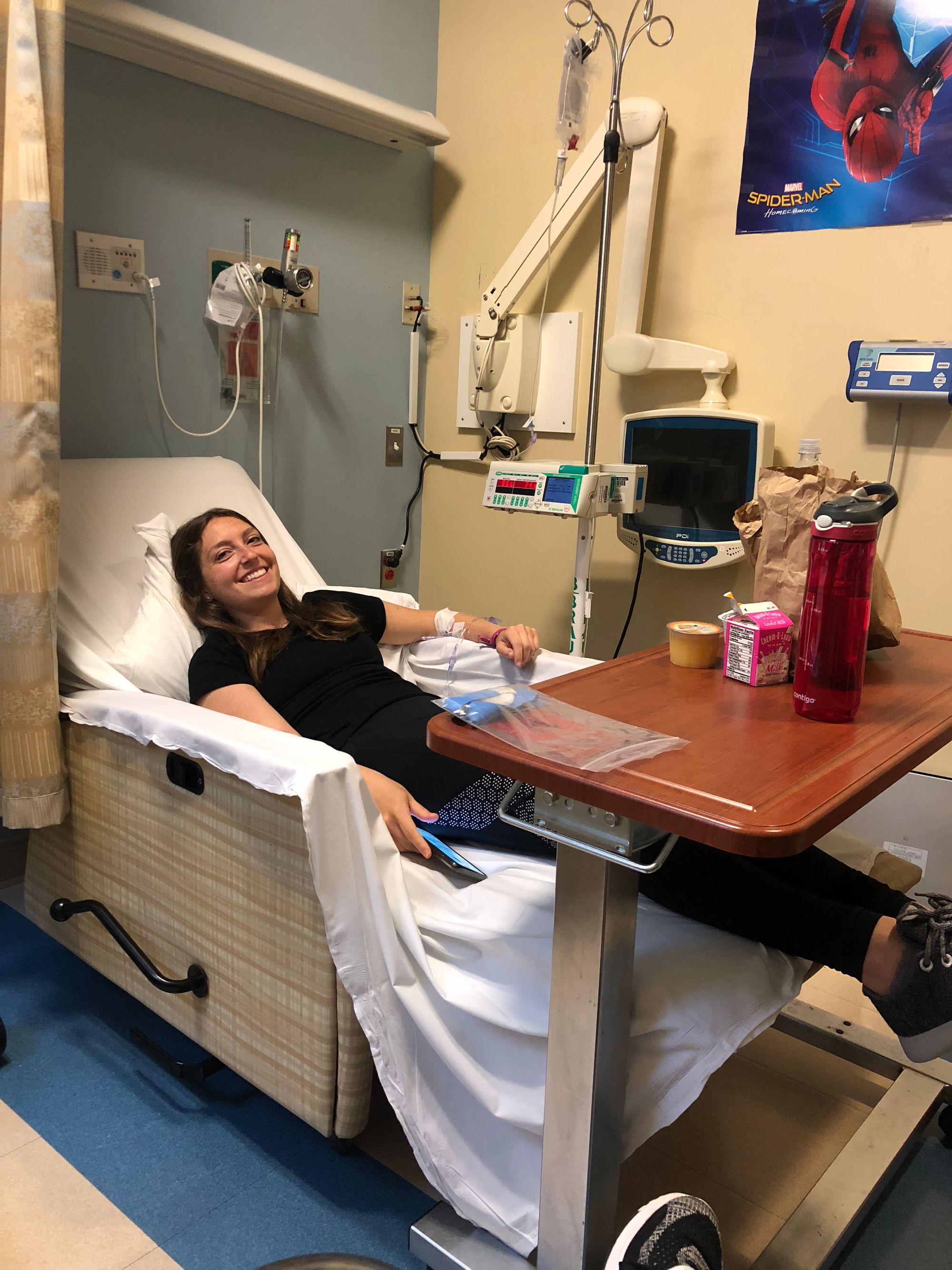
While the diagnosis felt right, Jamie wanted to ensure it was 100% accurate and felt strongly that a second opinion was essential. As an empowered patient, she researched Gaucher specialists and booked an appointment with a leading physician in lysosomal storage diseases in New York City. The doctor confirmed the diagnosis and explained to Jamie the lifelong treatment options available. Jamie decided that the best option would be ERT (enzyme replacement therapy), an IV infusion given bi-weekly that would last a little over an hour dependent on the individual to provide the missing enzyme that helps break down the excess fat being stored in bones and organs.
One of the treatment options caught Jamie’s attention - a clinical trial for an intravenous drug that had already been approved and on the market for several years. The company was running a clinical trial to show that over the course of two years, the therapy improves bone density, thereby improving symptoms of osteoporosis, which Jamie had developed in her spine. Jamie felt comfortable with the idea that the drug had already been on the market for many years and was encouraged by the fact that the treatment would be free for two years, a critical consideration given that the therapy is a lifelong treatment and is one of the most expensive drugs on the market.
On top of that, Jamie was thrilled to be receiving care under one of the top physicians in the world for her condition:
“I felt like it'd be very beneficial to have that close relationship with a doctor who's so well versed in the disease and is spearheading a lot of research with the disease. I knew it would be a great way for me to create a relationship with her and be under close watch just in case anything did go wrong or I had questions.”
Jamie became the first patient that was signed into the clinical trial at the NYC study site in over two years!
The Trial Experience
Overall, Jamie has had a largely positive experience with the clinical trial that started in July 2018. The hospital administrators handle booking all of her appointments and the sponsor company provides an American Express card for her travel and meal expenses on infusion days. Everything ran very smoothly while getting infusions in the hospital, and initially, Jamie really appreciated the security of being in a hospital should something go wrong.
Once Jamie got more comfortable with the infusion process and how the medicine made her feel physically, she was eager to take the sponsor company up on its offer to bring the trial to her, via a home nurse. In January 2019, Jamie began getting her treatment from the comfort of her own home on a bi-weekly basis and would only have to visit the hospital once per quarter. She shared:
“Since starting home infusions, any waiting time was now in the comfort of my own home and I could now use my couch instead of a hospital chair. It started to feel like a part of a routine rather than feeling like I was seeking treatment for a disease. This disease doesn’t define me and being at home makes this feeling ring true.”
Everything was running smoothly, until the world was hit with the COVID-19 pandemic.
Navigating uncharted waters: participating in a clinical trial through the COVID Craze
On March 11, 2020, COVID-19 was declared a global pandemic by the World Health Organization (WHO). Jamie had a major milestone appointment for the clinical trial scheduled for March 13, 2020. After reaching out to express her concern about traveling to the hospital in the wake of the pandemic, the appointment was cancelled and she was able to get her infusion at home.
While she was able to get the infusion this time, Jamie knew she still had many more treatments to go and it was clear that the COVID-19 pandemic wasn’t ending anytime soon. This resulted in high anxiety and uncertainty, but the organized event planner in Jamie kept her forging forward and advocating to ensure she could get her treatment on time.
She regularly communicated with her care team, brainstorming ideas to ensure she could stay in the trial. She was so dedicated to staying in the trial that she even told her doctors she would be willing to move to Boston for a few months where she has family if it meant staying on the therapy and in the study until it was completed.
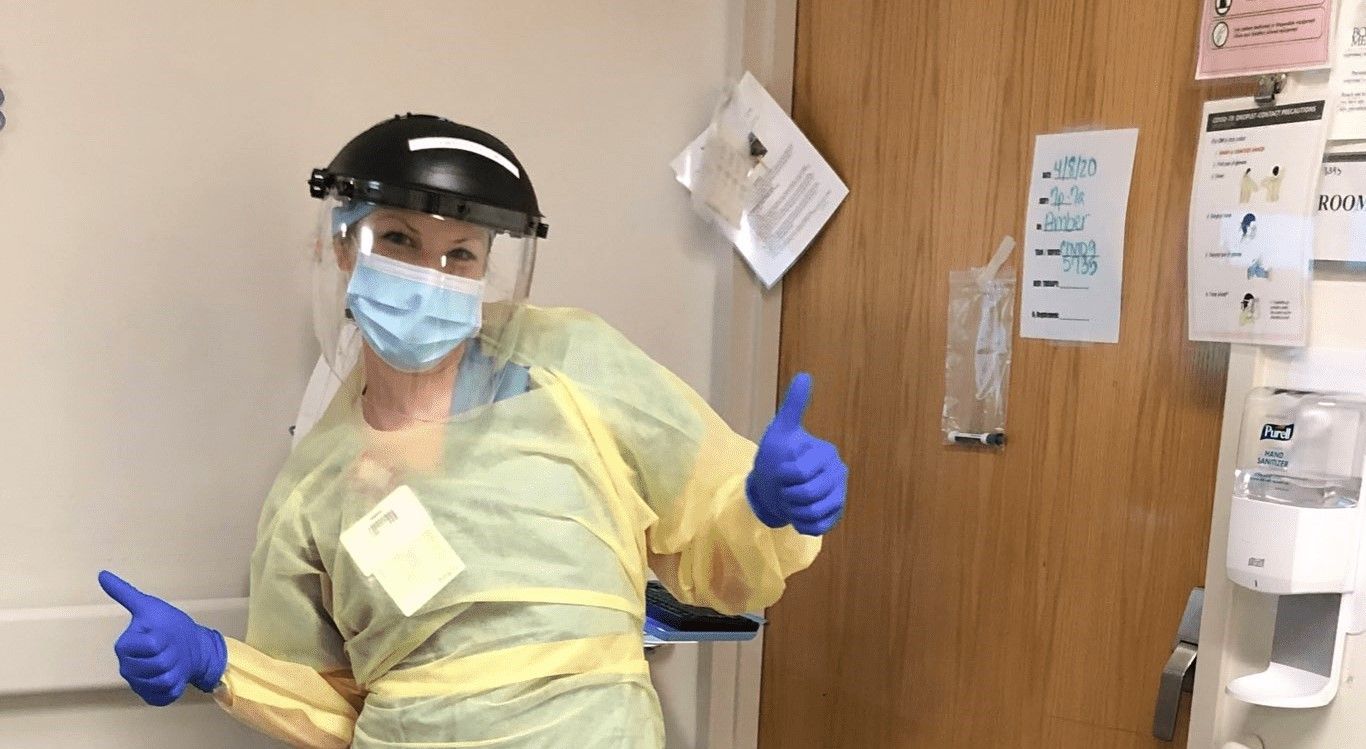
After her March infusion, Jamie’s home nurse who was coming to her apartment since January 2019 was moved to a COVID ICU unit within her hospital, meaning she would no longer be able to administer the infusion.
Jamie took matters into her own hands and advocated relentlessly to get her infusion scheduled despite a slew of unclear answers and breakdowns in communication. After many phone calls, emails, and spiked stress levels, the trial sponsor was able to identify a new nurse to come to her apartment that had not been in contact with any COVID patients and was not working on the frontlines.
She now has a nurse that commutes five hours each way to administer the infusion every other week (THANK YOU HEALTHCARE PROVIDERS!) and Jamie is hopeful she will be able to stay on the infusion through the end of her study in June.
Advice to fellow clinical trial participants in the wake of COVID-19
When asked what advice Jamie has for fellow patients participating in a clinical trial through COVID-19, she shared:
“There’s a lot of uncertainty, but try to stay positive. You might have to get creative with your solution. Be proactive. Ask to speak to your doctor and/or the nurse managing your clinical trial and be sure to come prepared with a list of questions. Persistence is key in this situation. You have to be your own advocate. Don’t think you are bothering someone by emailing them, calling them, and leaving them messages. You need to do what’s best for yourself. You know your body best. Think about what questions you want answered, not just for now, but for the future.”