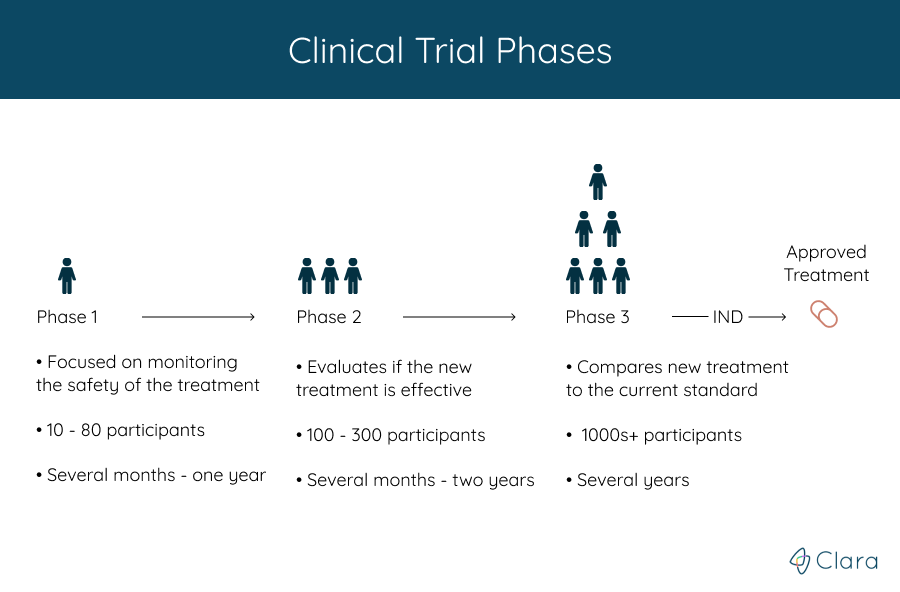
Clinical trials are run in multiple steps, called phases, that build on one another. Each phase helps answer different questions about the new treatment.
While the treatment’s safety and efficacy is monitored throughout each phase, the phase that a clinical trial is in roughly represents how much is known about the treatment that’s being studied.
The phase that a clinical trial is in can be a factor to consider when finding and deciding between different studies you might want to participate in.
You're never required to participate in all phases, and in most cases, you can join at any phase. For example, you could enroll in the phase 3 clinical trial of a specific treatment, even if you didn’t participate in its phase 2 clinical trial.
Note: While both cancer stages and clinical trial phases use the same numbers (1,2,3, and 4), the two numbers are different. Every trial has a different set of criteria that determines who can participate in the study, but the stage of cancer doesn't necessarily match which clinical trial phase is a good fit.
Phase 1: Is The New Treatment Safe?
Phase 1 clinical trials are usually the first to involve people, and help doctors learn if a new treatment is safe. Most often, the treatment is administered to a small group of healthy individuals, with the primary goal of testing for any major side-effects and safety issues, as well as determining the ideal dose.
Additionally, during this phase investigators seek the most effective and safest ways to administer the medication, whether that is orally, intravenously, or via other administration routes.
In certain cases, the treatment in the phase 1 clinical trial may be tested on volunteers who already have the condition. If you join a phase 1 clinical trial, you could be one of the first people to get a promising new drug or treatment.
In this clinical trial phase, the medical team collects information on:
- The most common side effects
- How your body responds to the treatment
- What dose of the treatment works the best (in the case of a drug)
The first few people in a phase 1 trial often get a very low dose of the treatment and are followed closely. If there are only minor side effects, the next few participants might get a higher dose. This continues until doctors find the dose that's most likely to work well.
Phase 1 clinical trials last from several months to a year, and usually have 10 to 80 participants. All information gathered from the phase 1 clinical trial helps researchers design the phase 2 study.
Approximately 70% of drugs move on to phase 2 trials, according to the FDA.
Phase 2: Does The Treatment Work?
If the phase 1 clinical trial shows the treatment is reasonably safe, it moves on to a phase 2 clinical trial to see if it works for treating the condition as well as to further assess its safety.
A larger group of 100-300 participants typically receive the amount of treatment that was found to be the most effective in the phase 1 trial. Phase 2 trials usually last over the course of several months to two years, and often new combinations of drugs are tested.
Although phase 2 clinical trials observe a bigger participant group than in the earlier phase, the size of the group is still not large enough to determine the overall risks and safety of the medication that’s tested. However, the data that is gathered during Phase 2 is then used by investigators to build out the methods for trials in later phases.
In some trials, the patients participating will receive different treatments. For example, one group could receive the FDA approved treatment that they would be receiving if they didn't participate in the clinical trial, and another would receive the new treatment that's being studied.
The response the doctors look for in patients depends on the goal of the treatment. For example, in a cancer clinical trial, it may mean the cancer disappears completely, or that patients on the new treatment live longer than they would have been expected to without the treatment. In other studies, especially for chronic conditions, the benefit may be an improved quality of life.
If the treatment seems to be effective for treating the condition, the study will likely move on to a phase 3 clinical trial.
The FDA approximates that 33% of medications make it to phase 3.
Phase 3: Is The Treatment Better Than What's Currently Available?
Phase 3 clinical trials are much larger than the ones conducted in Phase 1 or Phase 2. These trials involve hundreds or even thousands of participants, and are often conducted in many regions around the globe.
Treatments that have been shown to work in phase 2 are evaluated again and on a larger scale, prior to being approved for general use. Doctors compare the new treatment with the best treatment known today (also called the "standard treatment").
To do this, they need to assign people to different groups as part of the clinical trial. The patients in each group get a specific treatment, and the treatments are different between each of the groups. This helps them to see if the new treatment works better than the standard treatment, if it has fewer side effects, or both.
Because doctors don't know which treatment is better yet, study participants are often assigned to the groups randomly, and neither the doctors nor patients know which treatment they're on. Placebos may be used in some trials, but are rarely used alone if there's already an approved treatment that has been shown to work.
These trials can take many years, and if the results show that the treatment works well, the research team can then apply for FDA approval to make the treatment available to the general public.
In the case that the medicine is approved, regulators will decide how it would be used, as well as which patients would qualify to receive the new treatment. This will be determined based on the evidence that is gathered through all clinical trial phases of the medication.
Between 25-30% of medications move on to phase 4.
Getting The Treatment FDA Approved
When phase 3 clinical trials (or sometimes phase 2 trials) show a new drug is more effective and safer than the current standard treatment, a New Drug Application (NDA) is submitted to the Food and Drug Administration (FDA) for approval. The NDA, which includes data from all the pre-clinical and clinical studies, is reviewed by the FDA.
Based on the review, the FDA decides whether or not to approve the treatment for patients with the condition the drug was tested on (to be used in a different condition, additional clinical trials have to be done).
If the FDA believes that more evidence is needed to show that the new treatment's benefits outweigh its risks, it will ask for more information or even require that more studies to be done. If approved, the new treatment often becomes a standard of care, and newer treatments must often be compared to it before being approved.
In certain instances, the FDA approval of a new medication is accelerated. Accelerated Approval can be granted to medications that show promise in treating severe or life-threatening conditions, and may provide additional benefits over what is currently available.
After the medication becomes available on the market, the drug manufacturer must conduct post-marketing trials to confirm the therapeutic benefit of the drug. If later trials fail to show the predicted clinical benefits, the FDA may retract its approval of the new medication.
Phase 4: What Else Can We Learn About The Treatment?
Even after being approved by the FDA, treatments are still often watched for a long period of time in phase 4 studies. Although the medicine has been tested in thousands of people, additional studies can help show more about the treatment, such as very long term side effects. These studies may also look at other traits of the treatment, such as quality of life or cost effectiveness.
For example, a stem cell treatment may get FDA approval because it was shown to improve the function of individuals with knee osteoarthritis. But the medical teams may want to know if there are any rare side effects that haven’t been seen yet, or side effects that only show up after a person has had the therapy for a long time. These types of questions can take many years to answer, and are often answered in phase 4 clinical trials.
Phase 4 studies are generally the safest type of clinical trial, because the treatment has already been studied for a long time, and might have already been used in many people. While you can access the treatments in a phase 4 trial without enrolling in the study, participating can allow you to access an already FDA approved treatment at little to no cost, since the sponsor of the study will typically cover the cost of the therapy completely. Additionally, volunteering can help doctors learn more about the treatment and help patients in the future.
What Clara Does
Clara Health was established to help patients match with relevant clinical trials & research studies. We believe that all patients should have the power to access the most advanced healthcare available and our goal is to make this process as seamless as possible.
Want to see what Clara can do for you?
- Sign up for Clara Health (FREE)
- Instantly filter through over 50,000 clinical trials to find the studies that are a fit for you.
- If you’d like a helping hand, we can walk you through the process of finding and applying for clinical trials.





