Clinical trials are an essential part for finding the best treatments to fight diseases that work to decrease the quality of our human lives. They allow researchers to discover new methods for detecting, diagnosing, as well as reducing the chances of developing illnesses and diseases.
Trials give us the opportunity to study how medications work or don’t work in humans, and insights that cannot be simply observed in labs. They also give doctors an understanding of the potential benefits and risks/side effects of treatments, allowing them to make better decisions when deciding on a treatment for their patients.
Before a treatment can be approved for everyday use and made accessible to most patients, the U.S. Food and Drug Administration (FDA) requires that a potential therapy’s safety and efficacy be widely evaluated in a large group of human volunteers.
These studies, called clinical trials, are the last stage in the clinical research process before a treatment, such as a new drug, can be made available to patients. Once a treatment is deemed as helpful and safe, the manufacturer earns the permission to produce the drug or medication for public use.
It’s important to note that while researchers can predict the results of a clinical trial, the actual outcomes can never be predicted with 100% certainty. If this was the case, there wouldn’t be a need for clinical trials!
Individuals need to fully understand the implications of taking part in a trial, because there will always be an element of risk involved, as participants may experience negative side-effects. However, clinical trials are conducted in multiple phases, and the level of risk can be greatly reduced by entering a study that showed promise in being safe in earlier tests.
For example, if participating in a phase 3 clinical trial, the benefits and side effects of the treatment can usually be accurately hypothesized, as there is evidence from phase 1 and phase 2 clinical trials that shows how the new drug or medicine affected the participants.
While there have been cases of clinical trial participants experiencing negative side-effects during testing, billions of people have been helped from the discovery of new treatments, medications, and medical knowledge.
Clinical trials follow specific plans (protocols) developed by medical specialists to answer questions like:
- Does the treatment cause fewer side effects than the treatments used today, without being any less effective?
- Does it improve the quality of life for patients with the condition?
- Does the therapy work for people who aren’t being helped by the options that are currently available?
While the entire process to approve a new treatment lasts at least 10-15 years on average, participating in a trial can give patients access to therapies earlier than they would normally be available.
Clinical Trials: How Does A Treatment Get Qualified For A Trial?
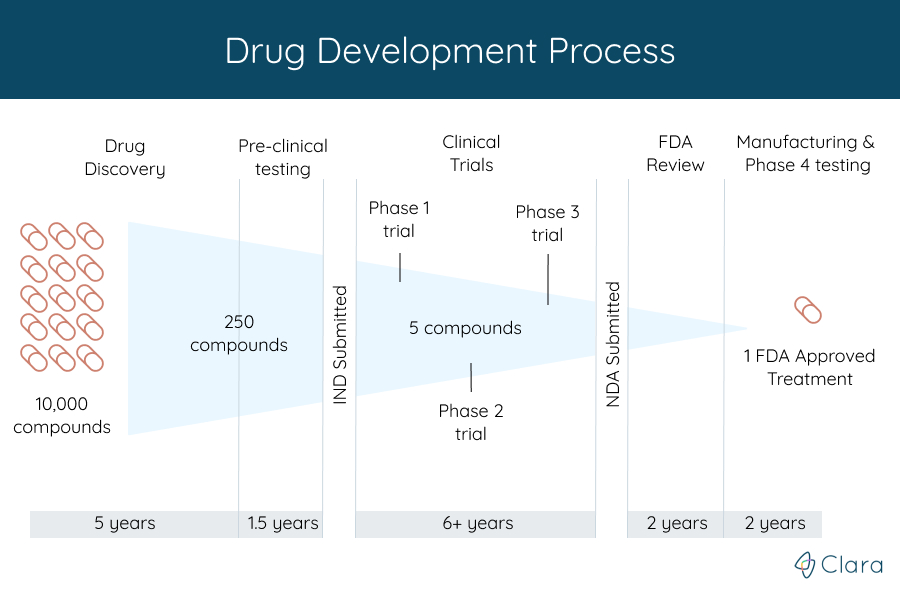
Before any clinical trial starts, the organization funding the study, also known as the trial's sponsor, is required to submit an Investigational New Drug (IND) application to the FDA.
This application includes the data they've collected from animal studies to show that the researchers have done the needed steps before starting a clinical trial. It also includes information about how the drug is made, what's in it, and how the clinical trials will be done.
With new drugs, for example, the trials are started only after treatments discovered in labs have gone successfully through years of chemical and biological studies.
Throughout the trial, the FDA and other organizations continue to make sure that patient safety is the highest priority. Of the many potential treatments tested in these early stages, very few are promising enough to enter into a clinical trial.
Yet, clinical trials can (and often do) fail because not enough people volunteer to participate, which increases the time it takes for new treatments to become available. That’s why volunteers who participate in trials play an extremely important role in allowing new treatments to reach patients.
The Different Types of Clinical Trials
Clinical trials, also known as interventional studies, directly evaluate the impacts of a specific treatment or preventative measure on a disease.
At least one group of participants in the study will receive a treatment (also called an "intervention") so that the researcher can evaluate its effects. There are many different types of studies, each unique in their treatments and goals.
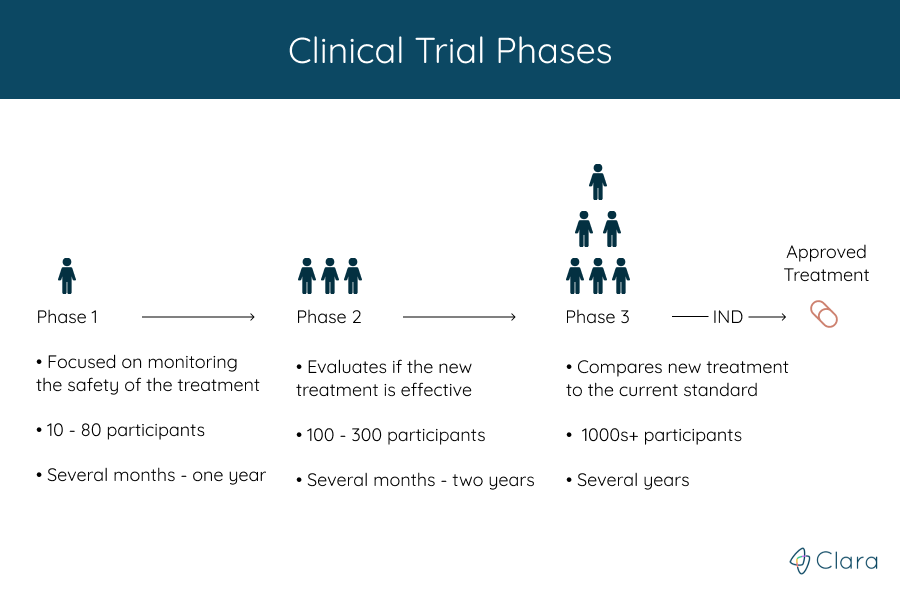
Trials are run in multiple steps, called phases, that build on one another. Each phase helps answer different questions, but the treatment’s safety and efficacy is monitored throughout the entire process. What phase a clinical trial is in gives an estimate for how much is known about the treatment being studied.
There are a total of four phases:
- A phase 1 trial is done to find if a new treatment is safe for people
- Phase 2 trials tell doctors more about how well the treatment works
- In a phase 3 trial, the new treatment is compared with a standard treatment that is given to patients today
After the first three phases, if the treatment is determined to be safe and effective, the FDA approves it for clinical use and continues to monitor its effects. Phase 4 studies look at treatments that have already been approved by the FDA.
There are a variety of new treatments being studied for different conditions, but some common types include:
- New drugs or surgeries
- Different ways of using existing treatments, such as testing if a drug that treats one condition works well for treating a different condition
- Medical devices (such as a pacemaker)
- Natural treatments, such as herbal remedies
- Changes in daily life, such as exercise, diets, or counseling
Observational Studies
Observational studies are clinical research studies that don’t test a new treatment, but instead follow a patient’s health condition and medical history to learn how it might be treated.
These studies have a wide variety of goals, like discovering risk factors for disease development or progression (called natural history studies), geographic or genetic correlations to diseases, or improving current treatment routines. Patients will usually continue their normal care routine and treatments when participating in an observational study.
Expanded Access Trials
Lastly, there are trials known as 'expanded access' studies. Expanded access, also known as “compassionate use,” provides a way for patients with a serious medical condition to access a treatment being tested in a clinical trial that they would not be eligible to participate in otherwise.
Participating In A Clinical Trial
A clinical trial can provide the best treatment option for you as you manage your medical condition.
There are clinical trials available for almost every condition, in many different places around the world. If you take part in a clinical trial, you may get tests or treatments in a hospital, clinic, or doctor's office.
Trials can be the right choice for a variety of people, at a variety of levels of health. They can be right for people who have yet to find a treatment that works to treat their condition, or those looking for a treatment that might work even better than current options.
They can be right for people who want to consider all available treatment options, or for people who have no options from formally approved drugs alone. Trials can be right for a perfectly healthy person, a patient suffering with a serious diagnosis, or any state of health in between.
What’s most important when deciding if participation in a trial is the right choice is understanding all the potential risks and rewards, and talking to your entire care team to choose the best course of action.
Here are the main possible benefits and risks of participating in a clinical trial:
Possible Benefits
- Since the entire clinical trial process lasts 10-15 years on average, participating in a trial can give patients access to the newest treatments earlier than they would normally be available.
- These treatments are held to higher standards, since the ultimate goal is to make them better than current treatment options. For example, psoriasis treatments currently in trials phases aim to completely eliminate all signs of the condition, whereas most of the currently available options aim to simply reduce the number of symptoms experienced.
- Many patients report that the experience of enrolling in a clinical trial was similar to what they would have received without participating, but they got better care and received closer attention from the trial team.
- The cost of the treatment is typically covered by the study’s sponsor, and they may also provide additional compensation to participants.
- The trial may help scientists learn more about what treatments work, and help people in the future.
Possible Risks
- The new treatment may not work better than the standard treatment does for you.
- New treatments may have side effects that doctors do not expect or that are worse than those of the standard treatment.
- You might be required to make more visits to the doctor than if you were receiving standard treatment.
- Health insurance may not cover all patient care costs in a trial - this should be discussed with the study team before you decide to participate.
What Happens When A Study Ends?
Once a clinical trial is finished, data that was collected throughout the study is thoroughly analyzed. After understanding the findings, researchers draw conclusions about how effective the treatment in question is at achieving the desired objective (such as slowing the development of the disease, or reducing its symptoms), as well as if further testing is needed.
When a phase 1 or 2 clinical trial is finished, researchers also complete statistical analyses to determine the probability of success, which allows them to decide whether the clinical study needs to move on to the next phase, or if the treatment is ineffective or unsafe, which would result in the terminating any plans for further tests.
If you decide to participate in a clinical trial, information regarding the length of the trial as well as if you will continue getting the treatment when the trial ends, should be provided to you prior to the start of the study.
In case you have any specific questions, make sure you get them answered prior to joining the study by asking the study coordinators. It is important that you have all the information you need about the clinical trial you’re looking to join, to ensure that it is right for you.
About Clara Health
With over 50,000 clinical studies worldwide, participating in a trial is an opportunity to be part of medical breakthroughs that can change the lives of other patients in the future.
At Clara, we understand that clinical trials can seem daunting. Though we believe that the biggest barrier is simply accessing enough information to feel comfortable taking your health care into your own hands. That’s why we created Clara Guides – to walk you through every step of the journey.
Are you or a loved one interested in participating in a clinical trial? Let us know! We'll help you every step of the way.
Sign up for Clara now or learn more here about how Clara supports patients through the clinical trials process.